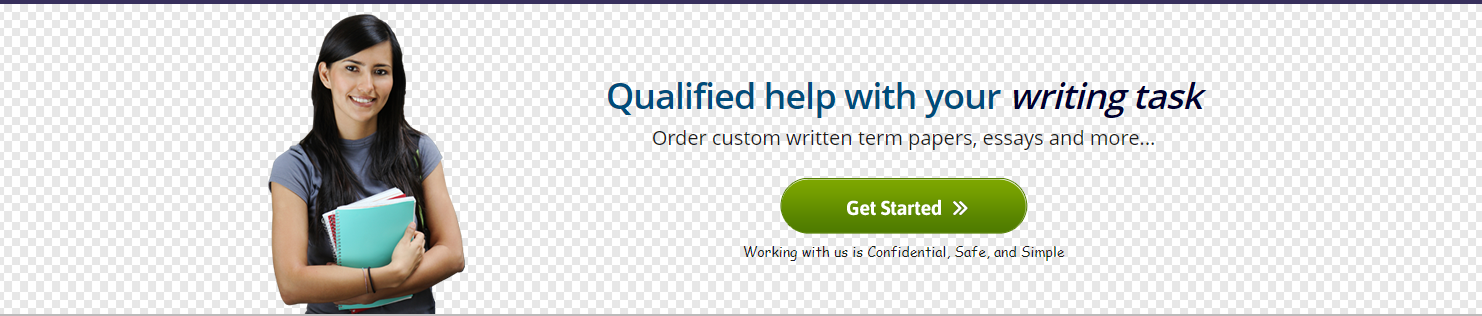
UCSD Physiology: Spectrophotometry of Hemoglobin Calculations • first graph using excel develop a graph clearly showing the absorption spectra of oxyhemoglobin , deoxyhemoglobin , and methemoglobin . -compare the ferric and ferrous states of iron . • second graph develop a standard curve for cyanmethemoglobin . Use standard curve and Beer’s law to estimate the concentration of an Unknown .Also turn in the calculations using beer’s law for the unknown, show work. 580
570
590
600
Absorbance (%) vs. Wavelength (nm) for Various Hemoglobin Samples
560
550
540
530
510
520
Wavelength 500
Oxy Hb
Cyanmet
1.77.73 1.811.95/1.08/1.07/1100/088/174.57 no
.99.5.12
130/127/137.37/145.90 1.631.28
30.34 136 143.621.66.52.97 1.85:20.19
Hb
Deoxy Hb
Transfer these data to an Excel spreadsheet and construct a line graph with wave
length as the independent variable and absorbance as the dependent variable. Be sure
your name is on the graph, and that the graph is on the same sheet at the data. All of
the lines should be on the same graph, so they can be easily compared. This graph
will be due at the beginning of the next lab session.
++
++++
Part II. Determination of Line of Best Fit (Linear Regression and Beer’s Law):
A. Beer’s Law: Beer’s Law states that the absorbance of a solution at a
particular wavelength of light is proportional to the concentration of the
absorbing solute molecule.
Aknown
(CA) (A Cu
Ak
Beer’s Law: A is proportional to C where A = absorbance and C = concentration
(Cx) (Au)=(A«) (C) = Aunknown
Cknown
Cunknown
From the above relationship, we conclude that it is possible to prepare solutions of various
cyanmethemoglobin concentrations, (our “known” values) determine their absorbances at a
fixed wavelength, and finally to construct a line graph by plotting the absorbance “A” vs.
concentration “C.” Theoretically, such a graph will show a linear relationship, with “A”
dependent on “C.” This line is known as a calibration curve. The calibration curve allows
us to roughly determine the absorbance, again at “peak” wavelength, of a solution of
unknown cyanmethemoglobin concentration, locate that absorbance on our curve, and
predict the actual solute concentration by interpolation.
Sample graph A: Calibration Curve
Abs.
Conc.
37
it should therefore, be possible to measure the concentration of hemoglobin in a sample
of blood by measuring the intensity of its color. In clinical practice the blood hemoglobin is
usually measured by this method. Colorimetric methods are complicated by the fact that
red blood cells contain different types of hemoglobin, and each different type absorbs light
maximally in a slightly different region of the visible spectrum. A more accurate answer for
unknown concentration can be determined by using your given data, and constructing
a linear regression equation, using the instructions below.
your
B. Linear Regression Equation and Determination of Unknown
Cyanmethemoglobin Concentration. The algebraic equation for a straight line is:
y = mx + b
where
Y-dependent variable
X = independent variable
b=y-intercept
m= slope of the line
Procedure:
You have already determined the “peak” wavelength at which cyanmethemoglobin absorbs
light. Locate this value. It may be expected to be close to 540 nm.
Determine the absorbance of the unknown and the four tubes of known
cyanmethemoglobin concentration, at 540 nm wavelength. Use the cuvette with 0 g/di
concentration (diluent only) as your blank for this determination. The known
concentrations are as follows:
0 g/dl, 5 g/dl, 10 g/dl, 15 g/dl, 20 g/dl (values in grams
cyanmethemoglobin per deciliter (100 ml) of solution.
Enter these data in the table below:
x values
(concentration)
(g/dl)
y values
(absorbance)
(%)
0
5
0
32
154
182
10
15
1:06
20
Enter these data into an Excel spreadsheet and generate a graph. Notice that the points do not
form an exact straight line. Generate a linear regression line to obtain the equation:
Go to the front table and select any one tube of “unknown concentration” from the tubes
provided. Take the absorbance (Y value) and record here:
(Y)=_0.52
-=10
Do not empty any of the cuvettes. Leave them in the test tube rack.
Now that you have determined your equation, you can plug in the Y value and solve for X,
which is the concentration of the unknown substance. Hand-write your calculations on your
printed graph. Be sure to show your work. Use the space below to practice. Use Beer’s Law to
check your answer.
X= ___g/dl
The hemoglobin determination graph as well as the linear regression graph is due at the
beginning of the next lab session.
Purchase answer to see full
attachment